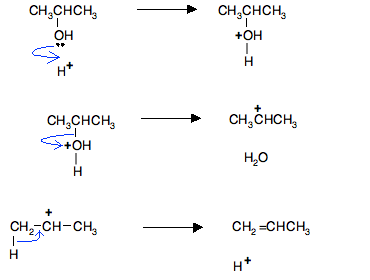
Isopropanol dehydration via extractive distillation using low transition temperature mixtures as entrainers – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science

PDF) Isopropanol dehydration via extractive distillation using low transition temperature mixtures as entrainers

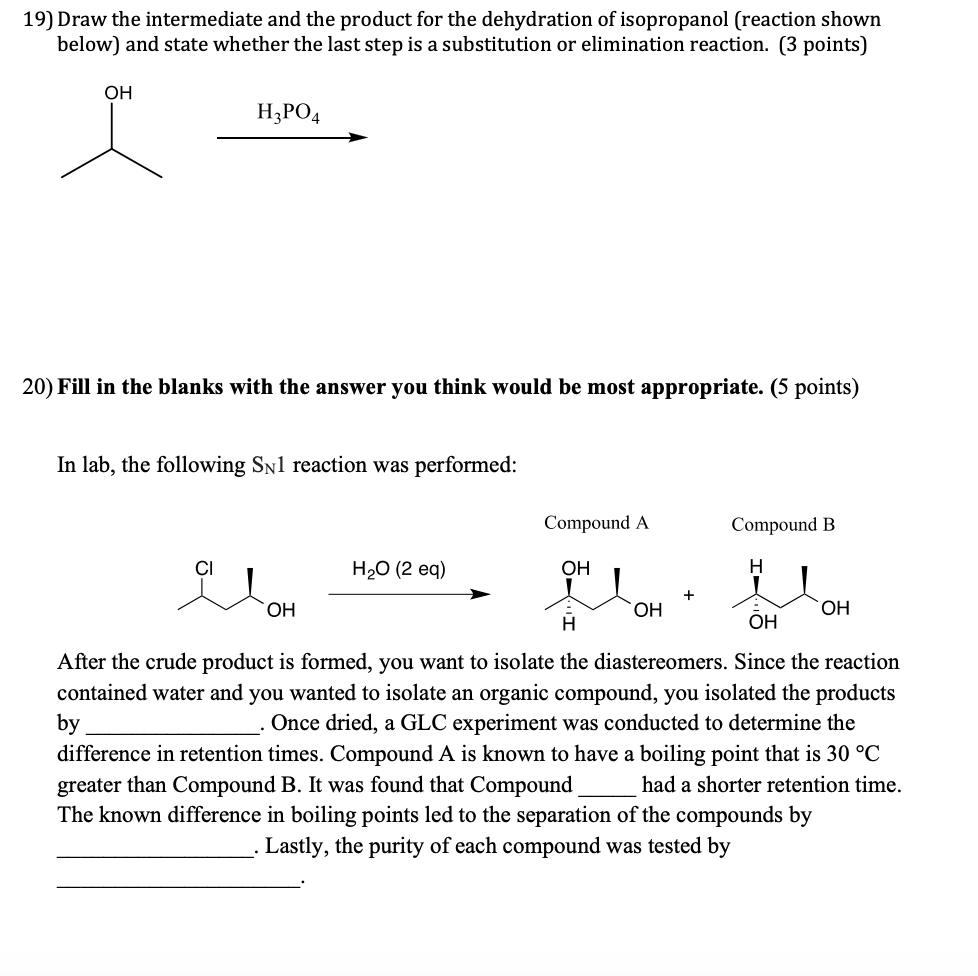
SOLVED:19) Draw the intermediate and the product for the dehydration of isopropanol (reactionoshosyn bedovthenatente whether the last step is a substitution or elimination reaction: (3 points) Oh H,PO4
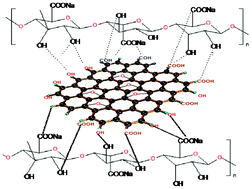
Graphene-loaded sodium alginate nanocomposite membranes with enhanced isopropanol dehydration performance via a pervaporation technique - RSC Advances (RSC Publishing)
![Dehydration of isopropanol to propylene over fullerene[C60] containing niobium phosphate catalyst: Study on catalyst recyclability - ScienceDirect Dehydration of isopropanol to propylene over fullerene[C60] containing niobium phosphate catalyst: Study on catalyst recyclability - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2468823119303025-ga1.jpg)
Dehydration of isopropanol to propylene over fullerene[C60] containing niobium phosphate catalyst: Study on catalyst recyclability - ScienceDirect

SciELO - Brasil - Catalytic Dehydration of 1-Propanol Over Silica Containing Sulfonic Acid Groups Catalytic Dehydration of 1-Propanol Over Silica Containing Sulfonic Acid Groups

Kinetic Studies of Heterogeneous Catalytic Isopropanol Conversion: Introduction to the Kinetics of dehydrogenation and dehydration of isopropyl alcohol on bi-metal oxides: Mansour, Rehab: 9783639677188: Amazon.com: Books

Novel approach to determination of sorption in pervaporation process: a case study of isopropanol dehydration by polyamidoimideurea membranes | Scientific Reports

Enhancing Dehydration Performance of Isopropanol by Introducing Intermediate Layer into Sodium Alginate Nanofibrous Composite Pervaporation Membrane | SpringerLink
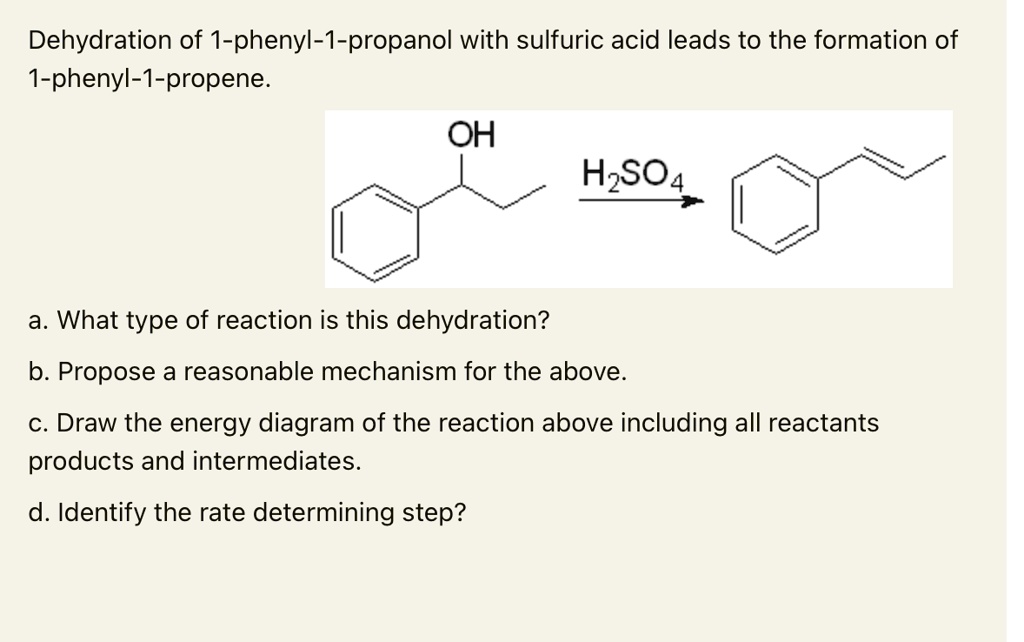
SOLVED:Dehydration of 1-phenyl-1-propanol with sulfuric acid leads to the formation of 1-phenyl-I-propene_ OH HzSQ4 a. What type of reaction is this dehydration? b. Propose reasonable mechanism for the above C. Draw the

The role of sodium and structure on the catalytic behavior of alumina: I. Isopropanol dehydration activity - PDF Free Download

Compensation in the isopropyl alcohol dehydration over sol–gel Al2O3–TiO2 oxides: Effect of calcining temperature - ScienceDirect



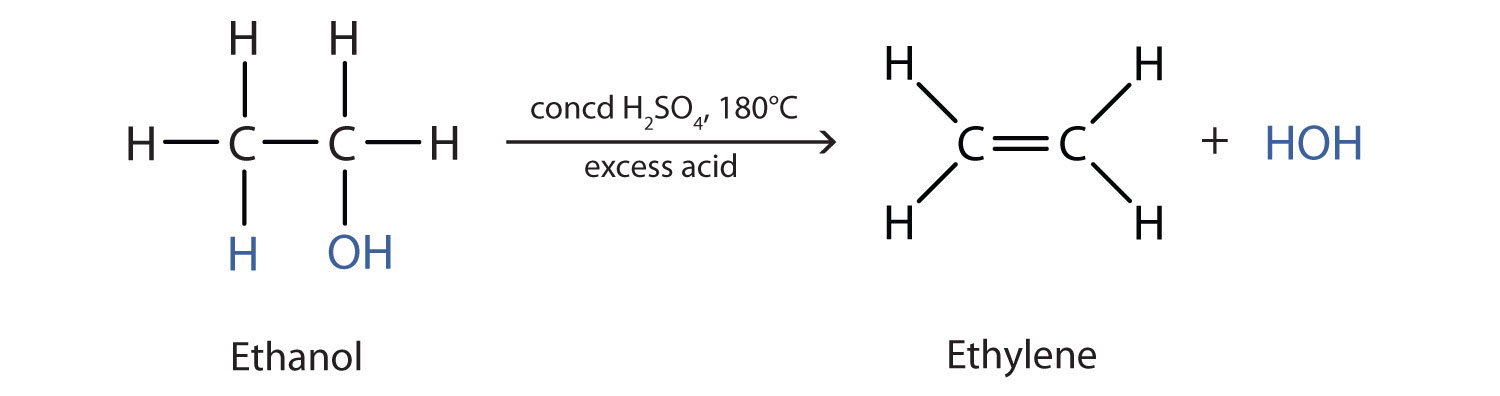



![PDF] Dehydration of Isopropanol and Ethanol by Pervaporation Technique | Semantic Scholar PDF] Dehydration of Isopropanol and Ethanol by Pervaporation Technique | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/af41383466a24ba7c79c04a47bce576608bb5beb/3-Figure2-1.png)